The benzaldehyde and acetone reaction mechanism unfolds as a captivating tale in the realm of organic chemistry, unveiling the intricate dance of molecules that leads to the formation of valuable compounds. This in-depth exploration delves into the chemical intricacies, applications, and safety considerations of this remarkable reaction.
1. Benzaldehyde and Acetone Reaction Overview: Benzaldehyde And Acetone Reaction Mechanism
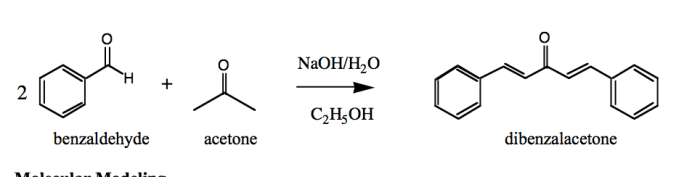
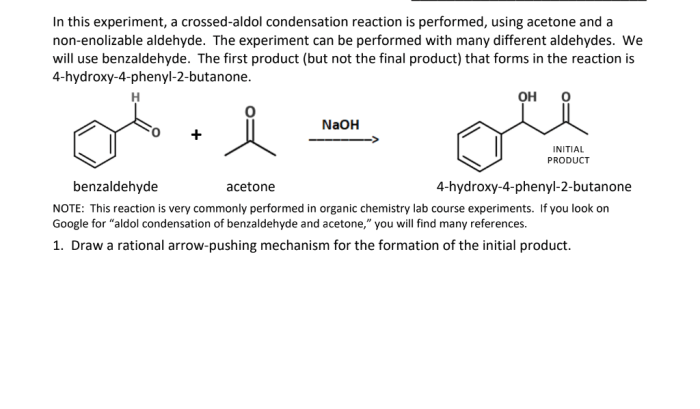
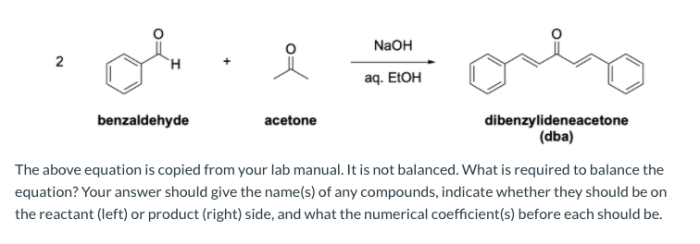
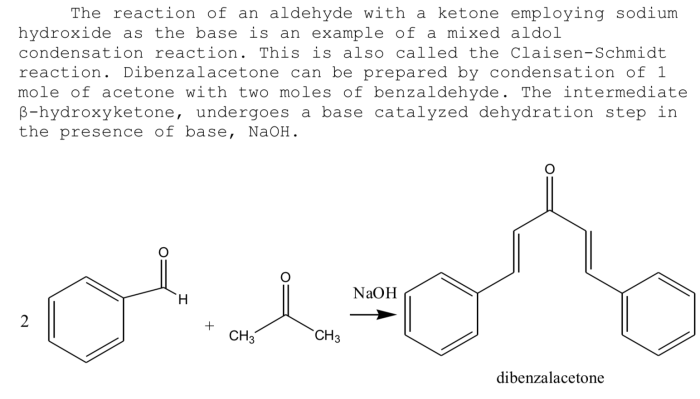
The reaction between benzaldehyde and acetone is a classic example of an aldol condensation, a fundamental reaction in organic chemistry. It involves the nucleophilic addition of the enolate ion of acetone to the carbonyl group of benzaldehyde, leading to the formation of a β-hydroxyketone intermediate.
This intermediate can then undergo dehydration to yield the final product, a conjugated enone.
This reaction is significant in organic chemistry as it provides a versatile method for the synthesis of various organic compounds, including pharmaceuticals, fragrances, and other chemicals.
2. Reaction Mechanism
The reaction mechanism for the benzaldehyde and acetone reaction proceeds through the following steps:
- Deprotonation of Acetone:Acetone is deprotonated by a base (such as sodium hydroxide) to form the enolate ion, which is a nucleophile.
- Nucleophilic Addition:The enolate ion attacks the carbonyl carbon of benzaldehyde, forming a tetrahedral intermediate.
- Proton Transfer:A proton is transferred from the tetrahedral intermediate to the base, regenerating the catalyst and forming the β-hydroxyketone intermediate.
- Dehydration:The β-hydroxyketone intermediate undergoes dehydration, catalyzed by acid or heat, to form the final product, a conjugated enone.
3. Reaction Conditions, Benzaldehyde and acetone reaction mechanism
The optimal reaction conditions for the benzaldehyde and acetone reaction vary depending on the specific catalyst and solvent used. However, some general guidelines include:
- Temperature:The reaction typically proceeds at room temperature or slightly elevated temperatures (40-60 °C).
- Solvent:Polar aprotic solvents, such as dimethylformamide (DMF) or dichloromethane (DCM), are commonly used.
- Concentration:The concentration of the reactants can affect the reaction rate and yield.
4. Applications of the Reaction
The benzaldehyde and acetone reaction is widely used in organic synthesis for the preparation of various organic compounds, including:
- Pharmaceuticals:The reaction is used to synthesize a variety of drugs, such as anti-inflammatory and analgesic agents.
- Fragrances:The reaction is used to produce fragrances and other scented compounds.
- Other Chemicals:The reaction is also used to synthesize other chemicals, such as dyes and polymers.
5. Safety Considerations
The benzaldehyde and acetone reaction involves the use of flammable and toxic chemicals. It is important to handle and dispose of these chemicals properly to minimize potential hazards.
- Flammable:Benzaldehyde and acetone are both flammable liquids. Keep away from heat and open flames.
- Toxic:Benzaldehyde and acetone are both toxic if ingested or inhaled. Use proper ventilation and wear appropriate personal protective equipment (PPE).
- Disposal:Dispose of chemicals and waste products according to local regulations.
Answers to Common Questions
What is the significance of the benzaldehyde and acetone reaction mechanism?
This reaction mechanism serves as a cornerstone in organic chemistry, providing a detailed understanding of the formation of important compounds and their applications.
How does the catalyst influence the reaction mechanism?
The catalyst plays a crucial role in facilitating the reaction, lowering the activation energy and accelerating the rate of product formation.
What are the key factors affecting the reaction rate and yield?
Temperature, solvent choice, and reactant concentration are critical factors that influence the efficiency of the reaction.